Aurobindo Receives FDA Approval for the line of OC tablets Aurovela™
Published: August 08, 2017
East Windsor, N.J. – Aurobindo Pharma Limited has received final approval from the U.S. Food and Drug Administration for Aurovela™ line of OC tablets. The Division of Bioequivalence has determined Aurobindo Pharma Limited’s Aurovela™ to be bioequivalent and, therefore, therapeutically equivalent to Loestrin® line of oral contraceptive products. Please see table below for each of the following product approvals.
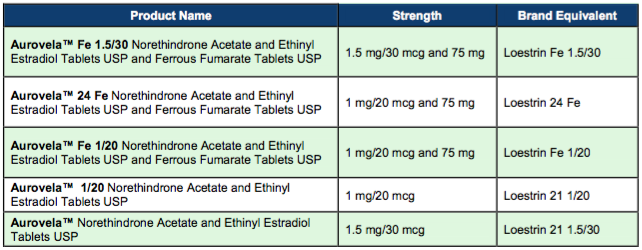
Aurovela™ line of tablets are oral contraceptives indicated for the prevention of pregnancy in women.
The combined products have an estimated market size of $315.9M for the twelve months ending May 2017 according to IMS*.
Aurovela™ line of OC tablets represents the latest addition to Aurobindo’s broad line of generic pharmaceuticals. Aurobindo’s product portfolio consists of 291 final approvals, including 36 tentative approvals. There are 102 additional products on file with U.S. FDA.
* IMS National Sales Perspectives: Retail and Non-Retail MAT June 2017
